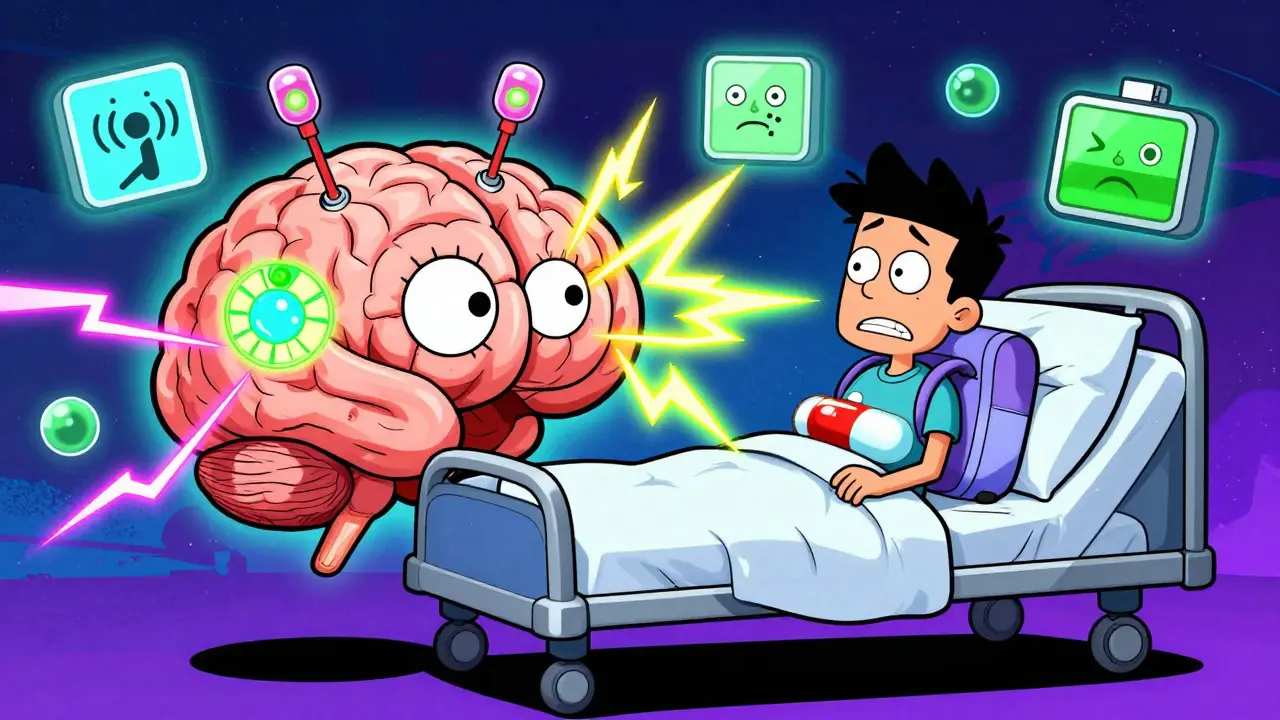
What Is Deep Brain Stimulation for Parkinson’s?
Deep Brain Stimulation, or DBS, is a surgical treatment for Parkinson’s disease that uses electrical pulses to calm overactive brain signals causing tremors, stiffness, and uncontrolled movements. It doesn’t cure Parkinson’s, but for many people, it dramatically reduces the worst symptoms-especially when medications stop working as well as they used to.
The system has three parts: thin wires (electrodes) placed deep in the brain, extension cables that run under the skin to the chest, and a small battery pack (called an IPG) implanted near the collarbone or abdomen. This battery sends steady electrical pulses to targeted areas like the subthalamic nucleus (STN) or globus pallidus interna (GPi), which control movement. Modern devices like Medtronic’s Percept™ PC and Boston Scientific’s Vercise™ Genus™ can even sense brain activity and adjust stimulation automatically.
Unlike older treatments like brain lesioning (which permanently destroy tissue), DBS is reversible and adjustable. If side effects appear, doctors can tweak the settings. If the battery dies, it can be replaced. This flexibility makes it a long-term option for people who still respond to levodopa but struggle with its side effects.
Who Benefits Most From DBS?
Not everyone with Parkinson’s is a good candidate. The best results come from people who meet specific criteria-and missing even one can mean poor outcomes.
- Diagnosed with idiopathic Parkinson’s: DBS doesn’t work well for atypical parkinsonism like progressive supranuclear palsy or multiple system atrophy. If your symptoms don’t improve with levodopa, DBS likely won’t help either.
- At least 5 years since diagnosis: This isn’t just a rule-it’s based on data. The CAPSIT-PD guidelines from 1999 still hold up. Early-stage patients rarely need surgery, and waiting ensures the diagnosis is solid.
- Strong response to levodopa: You should see at least a 30% improvement in your motor scores (UPDRS-III) when taking your medication. If you don’t, DBS won’t fix what the drug can’t touch.
- No major cognitive or psychiatric issues: If your memory is already declining (MMSE below 24 or MoCA below 21), or if you have untreated depression or hallucinations, DBS can make things worse. Neuropsychological testing is required before surgery.
Real-world data shows that 70-80% of people who meet these criteria get meaningful improvement. But if you’re still hoping DBS will stop your disease from progressing, you’re setting yourself up for disappointment. It treats symptoms-not the underlying brain degeneration.
STN vs. GPi: Which Target Is Right for You?
The two main brain targets for DBS are the subthalamic nucleus (STN) and the globus pallidus interna (GPi). Both improve movement, but they have different strengths.
| Feature | STN (Subthalamic Nucleus) | GPi (Globus Pallidus Interna) |
|---|---|---|
| Motor improvement | ~49% reduction in UPDRS-III | ~49% reduction in UPDRS-III |
| Medication reduction | 30-50% less levodopa | 0-20% reduction |
| Dyskinesia control | ~46% reduction | ~70% reduction |
| Cognitive side effects | Higher risk (word-finding, attention) | Lower risk |
| Battery life | Longer (less stimulation needed) | Shorter (higher output needed) |
Most people choose STN because it lets them cut back on pills-fewer side effects like nausea, sleepiness, and hallucinations. But if you already have mild memory issues or are worried about speech problems, GPi may be safer. If your biggest problem is severe dyskinesias, GPi gives better control. There’s no universal answer-it depends on your symptoms, lifestyle, and priorities.
The Evaluation Process: What to Expect Before Surgery
Getting approved for DBS isn’t quick. It takes 3 to 6 months and involves multiple specialists.
- Neurologist assessment: You’ll repeat your UPDRS-III motor exam-once off meds, once on. This confirms your levodopa response.
- Neuropsychological testing: You’ll spend 4-6 hours answering questions, doing memory tasks, and solving problems. This checks for hidden cognitive risks.
- High-resolution MRI: A 3T or higher MRI maps your brain to plan electrode placement. Some centers use specialized software to simulate the surgery.
- Team review: A neurologist, neurosurgeon, and neuropsychologist meet to decide if you’re a candidate. Some centers also include a physical therapist or speech pathologist.
- Insurance approval: Medicare and most private insurers require proof that you’ve tried and failed optimal medication therapy. This often means documenting 3-6 months of medication adjustments.
Don’t underestimate this step. Skipping any part leads to higher complication rates. A 2021 study in the Journal of Neurosurgery found that centers doing more than 50 DBS surgeries per year had 20% fewer complications. Choose a high-volume center if you can.
What Happens During and After Surgery?
The surgery itself takes 3-6 hours and is usually done in two stages, though some centers do it in one.
You’re awake during electrode placement so you can respond to questions. A frame is attached to your head, and tiny holes are drilled into the skull. Surgeons use microelectrode recording to listen to brain signals and confirm the exact spot. You might be asked to move your hand or count aloud while stimulation is tested. It’s intense, but most patients tolerate it well.
After surgery, you’ll stay in the hospital for 1-2 days. The battery is implanted a week or two later. Then comes the long wait: stimulation doesn’t start right away. It usually begins 2-4 weeks post-op, once swelling goes down.
Programming takes months. The first few visits are every 2-4 weeks. Settings are adjusted slowly-too much stimulation can cause muscle tightening, speech problems, or mood changes. Many patients need 6-12 months to find their best settings. Keep a symptom diary. Note when you feel stiff, shaky, or overly active. This helps your team fine-tune the device.
Real Stories: Benefits and Challenges
People who get DBS often say it gives them their life back-but not without trade-offs.
One man in his 60s, after STN DBS, went from 6 hours of OFF time daily to under an hour. His dyskinesias dropped by 90%. He cut his levodopa dose in half. But he started forgetting words mid-sentence. He needed speech therapy to get them back.
A woman in her 50s chose GPi because she had severe dyskinesias. Her movements became smooth. But she had to keep her medication dose high, which caused dizziness. Her battery lasted only 4 years-she had to have it replaced.
On forums like Reddit and Parkinson’s Foundation boards, common complaints include:
- “I thought DBS would fix my balance problems-it didn’t.”
- “Programming is frustrating. I had to visit 12 times before it felt right.”
- “My husband says I’m more irritable. I didn’t expect that.”
These aren’t failures. They’re normal. DBS works best for motor symptoms. It doesn’t fix freezing, falling, speech softening, or dementia. Managing expectations is half the battle.
Cost, Risks, and Long-Term Outlook
DBS costs between $50,000 and $100,000 in the U.S., but Medicare covers it. Private insurance usually does too-if you’ve met the criteria.
Surgical risks are low but real:
- 1-3% chance of brain bleed (stroke-like symptoms)
- 5-15% chance of hardware issues (infection, broken wire, lead shift)
- 1-2% risk of permanent side effects like weakness or speech problems
Battery life varies. Non-rechargeable models last 3-5 years. Rechargeable ones (like Percept™ PC) last 9-15 years. You’ll need a minor surgery every few years to replace the battery.
Long-term, 85% of patients still benefit 10 years after surgery. But axial symptoms-walking, balance, posture-only improve 20-30%. That’s why research is now focused on earlier intervention. The EARLYSTIM-2 trial is testing DBS in patients with just 3 years of Parkinson’s. Early results suggest it might prevent some decline.
What’s Next for DBS?
DBS isn’t stuck in the past. New tech is making it smarter.
Closed-loop systems like Medtronic’s Percept™ PC now monitor brain waves in real time. They detect abnormal beta rhythms (13-35 Hz) and adjust stimulation automatically. The INTREPID trial showed 27% better symptom control than traditional DBS.
Researchers are also exploring:
- Using Apple Watch data to track tremors and feed it back to the DBS device
- Genetic testing to predict who responds best (LRRK2 mutation carriers show better outcomes)
- Targeting non-motor symptoms like depression and anxiety
Still, the biggest barrier isn’t technology-it’s access. Only 1-5% of eligible patients get DBS. Many are never referred. Others wait until they’re too far gone. If you’re considering it, talk to your neurologist now-not when you’re falling or can’t get out of bed.
Is DBS Right for You?
Ask yourself these questions:
- Do I still respond well to levodopa?
- Do I have 5+ years of Parkinson’s?
- Are my biggest problems tremors, stiffness, OFF periods, or dyskinesias?
- Do I have memory issues, depression, or hallucinations?
- Am I ready for a long process with multiple appointments and possible surgeries?
If you answered yes to the first three and no to the fourth, you’re likely a strong candidate. If you’re unsure, ask for a referral to a movement disorders center. Don’t wait until you’ve lost too much independence. DBS works best when it’s a tool to preserve quality of life-not a last resort.