An Overview of Calcium Carbonate and Its Synthetic Production
Calcium carbonate is a chemical compound with the formula CaCO3, found naturally in rocks, shells, and the Earth's crust. It has a wide range of applications, including as a dietary supplement, antacid, and filler in various industries such as paper, plastic, and construction. While calcium carbonate can be obtained from natural sources, there is a growing demand for its synthetic production. This is mainly due to the need for a consistent and high-quality product that can meet specific requirements of various industries. In this article, we will delve into the world of synthetic calcium carbonate, exploring different methods of production and their respective advantages.
Production Method 1: Precipitated Calcium Carbonate (PCC)
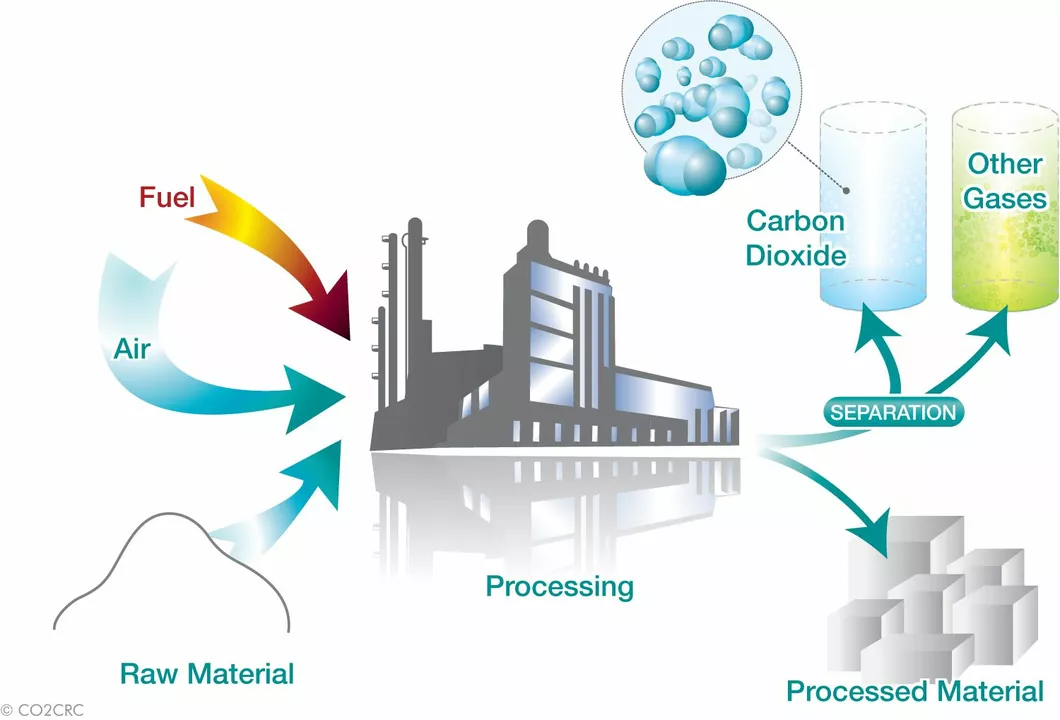
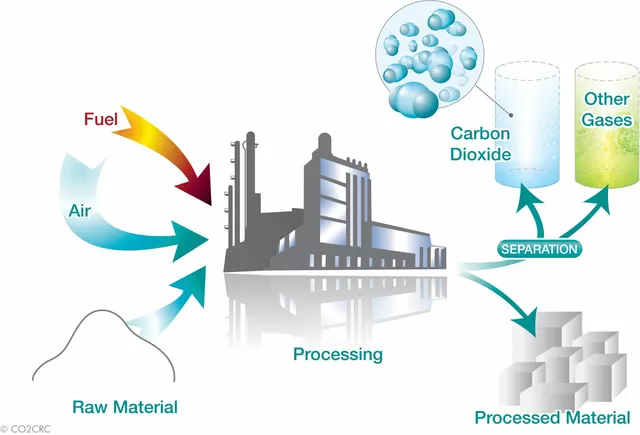
Precipitated calcium carbonate (PCC) is one of the most commonly used synthetic calcium carbonate production methods. This process involves the reaction between calcium hydroxide (Ca(OH)2) and carbon dioxide (CO2). Calcium hydroxide is commonly obtained by treating calcium oxide (CaO) with water, while carbon dioxide can be sourced from various industrial processes or natural gas deposits.
During the reaction, calcium carbonate is formed as a solid precipitate, which can then be filtered, washed, and dried to obtain the final product. The properties of PCC, such as particle size, shape, and purity, can be controlled by adjusting the reaction conditions, such as temperature, pressure, and the concentration of reactants.
This method is widely used in industries such as paper, plastics, and paints, where the specific properties of PCC are required. For example, PCC with a small particle size and high brightness is ideal for use as a filler in paper, as it improves the opacity and brightness of the final product.
Production Method 2: Solvay Process
The Solvay process is another method for producing synthetic calcium carbonate, primarily used for the production of soda ash (sodium carbonate) and calcium chloride. This process involves the reaction between sodium chloride (NaCl), ammonia (NH3), and carbon dioxide (CO2) in water.
During the Solvay process, calcium carbonate is formed as a byproduct, which can then be separated, washed, and dried to obtain the final product. This method is particularly advantageous for industries that require both soda ash and calcium carbonate, as it allows for the simultaneous production of these two valuable chemicals.
However, the Solvay process is less commonly used for the sole purpose of synthetic calcium carbonate production, as it generally results in a product with lower purity and less desirable properties compared to PCC.
Production Method 3: Carbonation of Calcium-Containing Waste Materials
Another method for producing synthetic calcium carbonate involves the carbonation of calcium-containing waste materials, such as industrial byproducts or waste shells. This process is considered an environmentally friendly and sustainable option, as it helps to recycle waste materials and reduce carbon dioxide emissions.
During this process, waste materials are treated with carbon dioxide, often under high pressure and temperature, to form calcium carbonate. The product can then be separated, washed, and dried to obtain the final product. This method can produce calcium carbonate with properties similar to those of PCC, depending on the reaction conditions and the quality of the waste material used.
Some industries, such as cement and construction, are increasingly adopting this method to produce synthetic calcium carbonate, as it helps to reduce their environmental footprint and improve their waste management practices.
Production Method 4: Electrochemical Processes
Electrochemical processes have also been explored as a method for producing synthetic calcium carbonate. These processes involve the use of an electric current to drive the reaction between calcium ions and carbonate ions, leading to the formation of calcium carbonate.
One example of an electrochemical process is the electrodialysis method, which uses a membrane to separate calcium and carbonate ions and force them to react under the influence of an electric field. This method can produce high-purity calcium carbonate with controlled particle size and morphology.
While electrochemical processes show promise for the production of synthetic calcium carbonate, they are currently less commonly used compared to other methods, such as PCC, due to their relatively high energy consumption and complex setup.
Choosing the Right Method for Your Application
In conclusion, there are several methods available for producing synthetic calcium carbonate, each with its own advantages and limitations. The choice of the most suitable method depends on various factors, such as the desired properties of the final product, the availability of raw materials, and the specific requirements of the industry in question.
For most applications, PCC remains the go-to method due to its versatility and ability to produce high-quality calcium carbonate with controlled properties. However, as industries continue to search for more sustainable and environmentally friendly solutions, alternative methods such as carbonation of waste materials and electrochemical processes may gain more traction in the future.
Ultimately, the key to selecting the right method for synthetic calcium carbonate production lies in understanding the specific needs of your application and carefully evaluating the benefits and drawbacks of each available method.
Also-can we PLEASE stop calling it ‘synthetic’? It’s not fake, it’s just made in a reactor instead of a cave. We’re not making synthetic love, we’re making synthetic filler for toothpaste. Chill.