Science and Technology – Simple Guides & Fresh Updates
If you’re curious about what’s happening in labs or on your phone, you’ve landed in the right spot. This page pulls together clear, bite‑size articles that explain real science and tech without the jargon.
How Calcium Carbonate Is Made – A Quick Look
Calcium carbonate is everywhere—from chalkboards to dietary supplements. The most common industrial route starts with calcium chloride mixed with sodium carbonate. When they meet, a white solid called calcium carbonate drops out of the solution.
The next steps are straightforward: filter out the solid, wash it to get rid of leftover salts, and dry it. The result is either precipitated calcium carbonate (PCC) or ground calcium carbonate (GCC), depending on how fine you grind it. PCC is used in paper coating, while GCC finds its way into toothpaste.
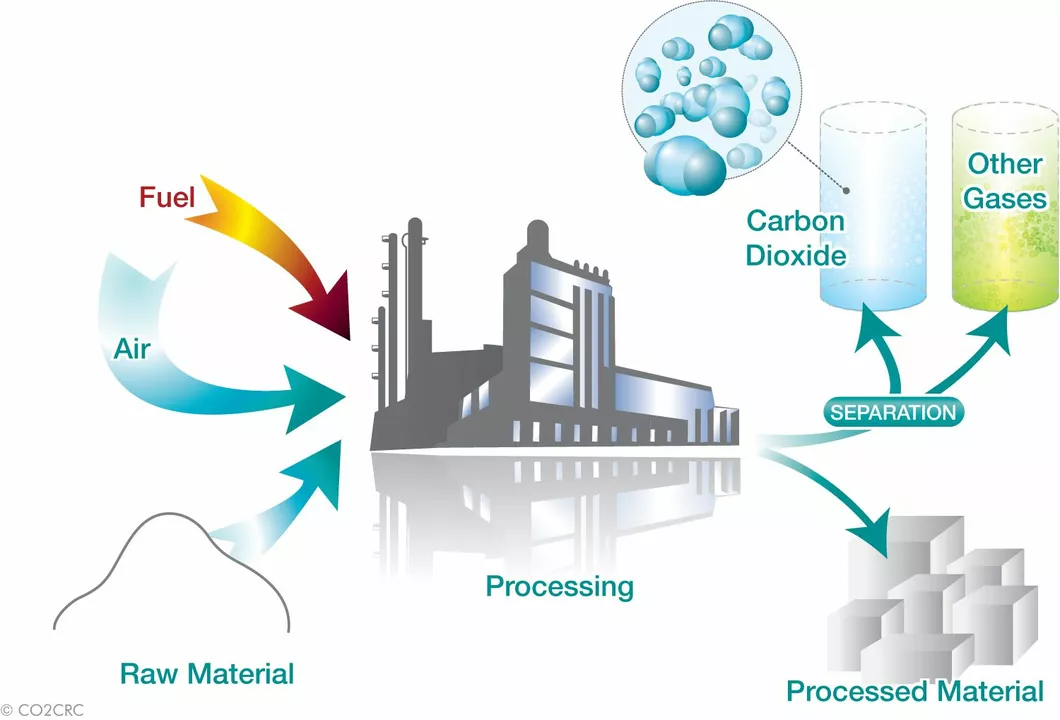
There’s a second method that starts with quicklime (calcium oxide). Add water and you get calcium hydroxide. Blow carbon dioxide through that mixture and the same white solid forms. This route is handy when you have cheap limestone on hand and want to capture CO₂ at the same time.
Why It Matters for You
You might wonder why a chemical recipe matters in daily life. The truth is, calcium carbonate helps keep your water clear, makes pills easier to swallow, and even improves soil for farming. Knowing how it’s made lets you spot greener options—like producers that recycle CO₂ instead of releasing it.
Beyond chemistry, the page covers tech topics that affect your smartphone, home gadgets, and online safety. Each article sticks to practical tips: How to extend battery life, what a software update really does, or why two‑factor authentication is worth the extra step.
We keep things short because you probably skim before you read. If something catches your eye, dive deeper with the full post—no need for a PhD to understand it.
Got a question about a new gadget or a lab process? Drop a comment and we’ll try to answer in plain language. Our goal is to turn confusing science into useful knowledge you can apply right away.
Stay tuned for fresh posts every week. Whether you’re a student, a DIY enthusiast, or just someone who likes knowing what’s behind the products you use, this corner of the site is built for you.
Liability and Indemnification in Generic Transactions Explained
Indemnification in commercial transactions is a legal mechanism that shifts financial risk between parties. Learn how it works, what clauses to demand, and how to avoid costly mistakes in contracts.
Whistleblower Laws: Protections for Reporting Violations
Whistleblower laws protect employees who report illegal or unsafe practices at work. Learn how California's 2025 rules strengthen protections, what retaliation looks like, and how to safely report violations without losing your job.
How calcium carbonate is produced synthetically
Calcium carbonate is a widely used compound, and its synthetic production is an interesting process. First, a reaction between calcium chloride and sodium carbonate takes place, which forms calcium carbonate as a precipitate. This is then filtered, washed, and dried to obtain a pure product. Another method involves reacting quicklime with water to create calcium hydroxide, which is then exposed to carbon dioxide gas to produce calcium carbonate. These processes allow for the creation of various forms of calcium carbonate, such as precipitated calcium carbonate (PCC) and ground calcium carbonate (GCC), which are useful in numerous applications.