Rxmedonline.com: Your Convenient Pharmacy Portal - Page 5
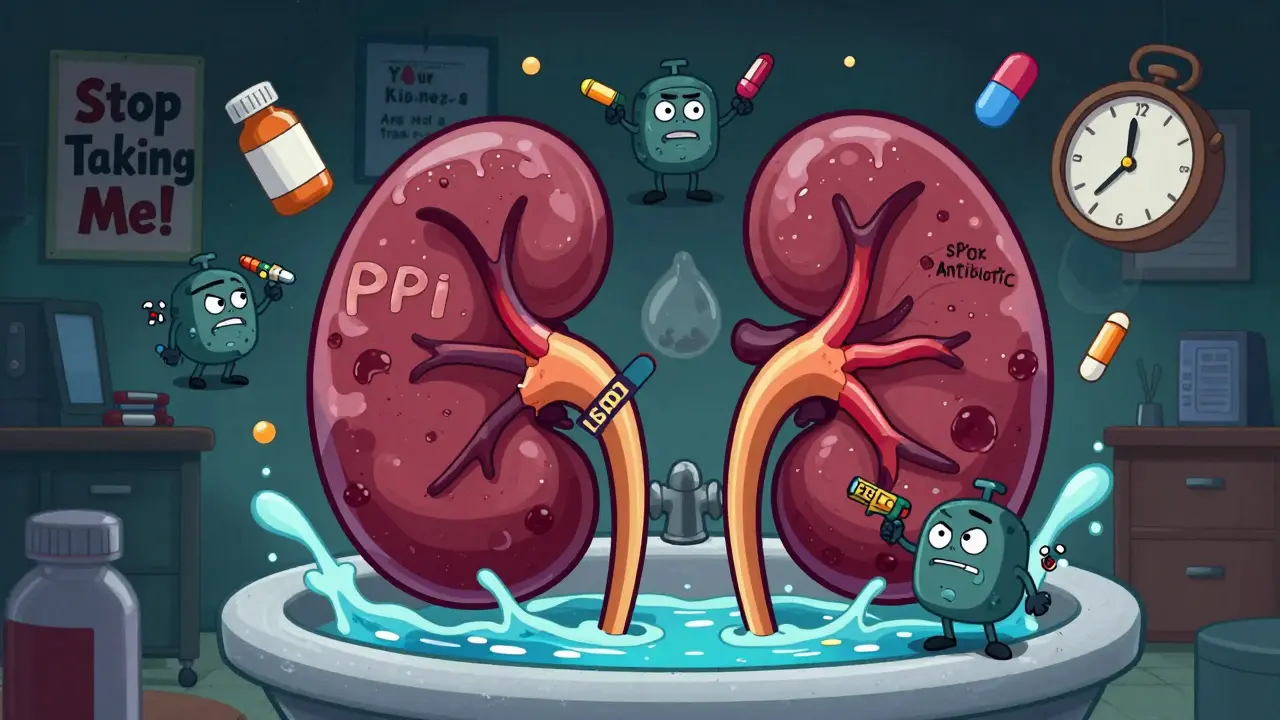
Acute Interstitial Nephritis: How Drugs Trigger Kidney Inflammation and What Recovery Really Looks Like
Acute interstitial nephritis is a serious kidney condition often caused by common medications like PPIs and NSAIDs. Learn how drug reactions trigger inflammation, why diagnosis is delayed, and what recovery really looks like based on real patient outcomes.
How FDA Ensures Generic Drug Quality During Manufacturing
The FDA ensures generic drug quality through strict cGMP standards, unannounced inspections, and rigorous testing. Every generic drug must match the brand-name version in safety, strength, and effectiveness - not by luck, but by design.
Vortioxetine and Nausea: How to Manage Early Side Effects and Stay on Track
Vortioxetine can cause nausea in up to 30% of users, but it usually fades within two weeks. Learn how to manage it with low-dose starting, ginger, food timing, and when to ask for help.
GLP-1 Receptor Agonists for Weight Loss and A1C Reduction: What You Need to Know
GLP-1 receptor agonists like Ozempic and Wegovy help lower A1C and promote significant weight loss by reducing appetite and slowing digestion. Learn how they work, who benefits, and what to expect.
Large Print and Accessible Prescription Labels for Low Vision: What You Need to Know
Learn how large print and accessible prescription labels help people with low vision take medications safely. Discover what standards apply, which pharmacies offer them, and how to get them - no doctor’s note needed.
Injectable Medication Shortages: Why Hospital Pharmacies Are on the Front Line
Hospital pharmacies are bearing the brunt of a growing crisis: sterile injectable drug shortages. With 226 active shortages in mid-2025, critical medications like anesthetics and chemotherapy drugs are vanishing - forcing staff to make impossible choices and delay life-saving treatments.
Parkinson’s DBS: How Deep Brain Stimulation Works and Who Is a Good Candidate
Deep Brain Stimulation (DBS) can dramatically reduce Parkinson’s motor symptoms for eligible patients. Learn who benefits most, how STN and GPi targets differ, what the evaluation process involves, and what to expect before and after surgery.
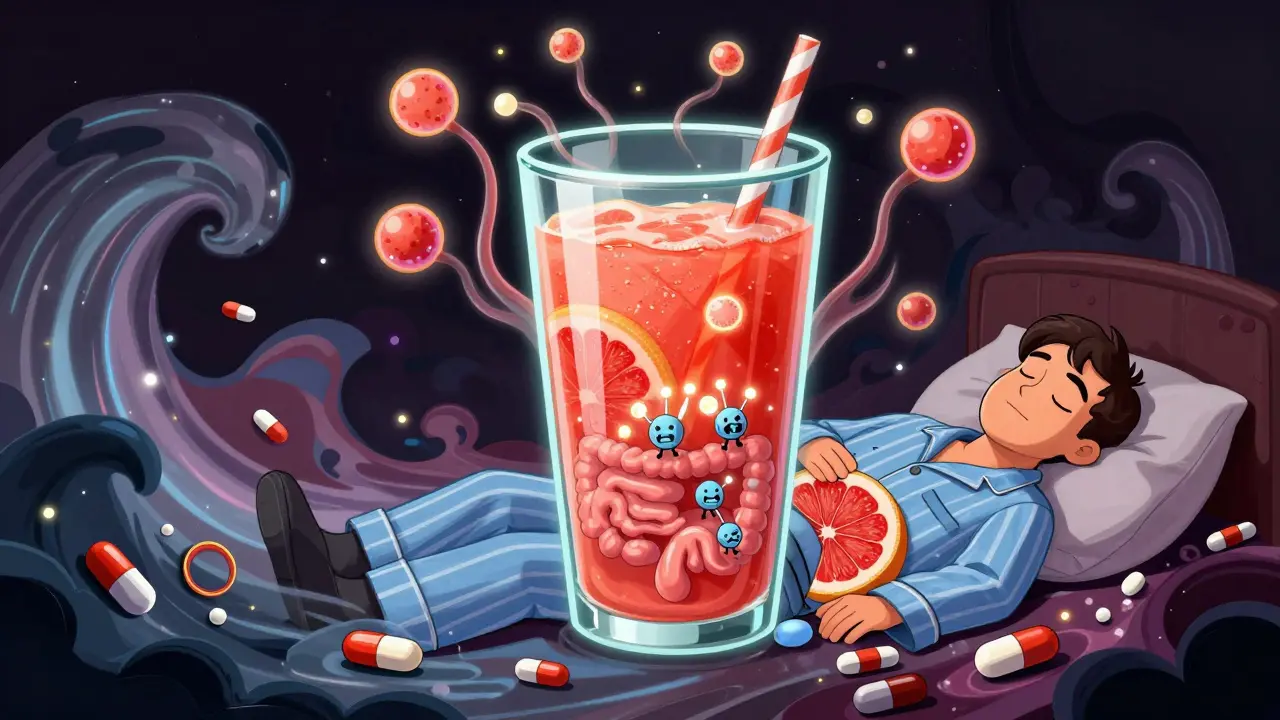
Grapefruit Juice and Simvastatin: What You Need to Know About Myopathy and Toxicity Risk
Grapefruit juice can dangerously increase simvastatin levels in the blood, raising the risk of muscle damage and kidney failure. Learn how much is unsafe, which statins are safer, and what symptoms to watch for.
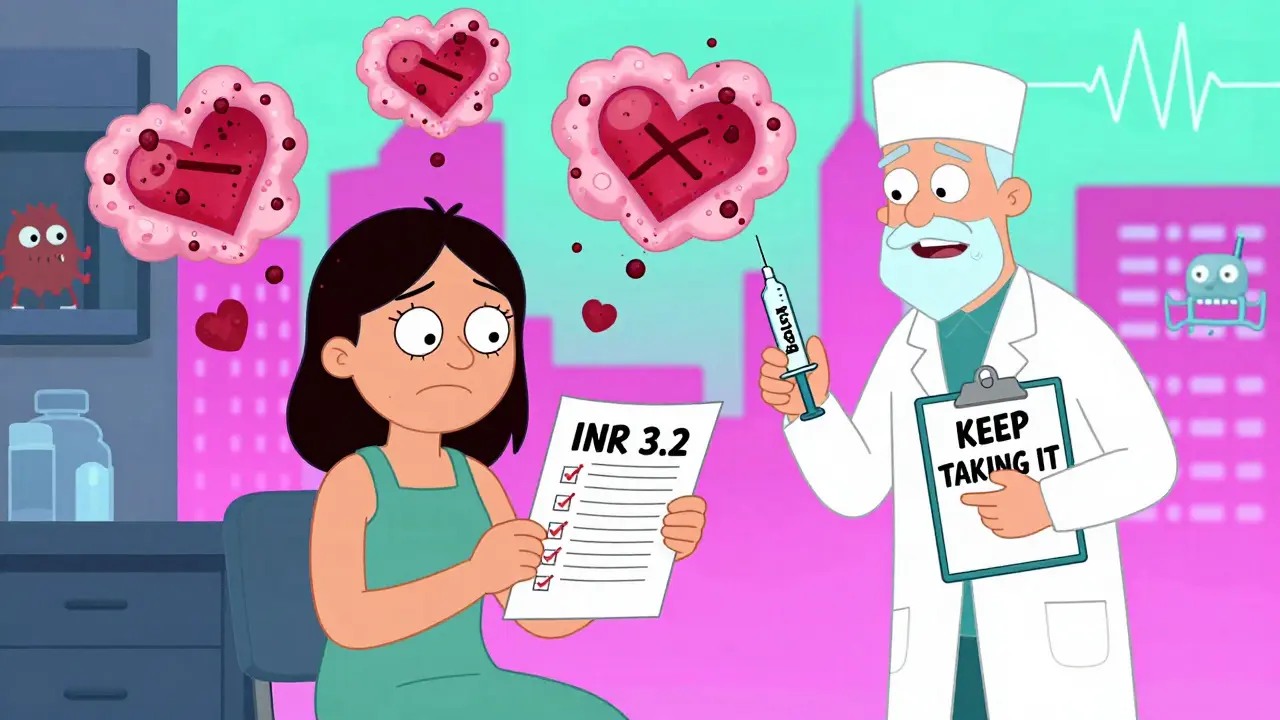
Cosmetic Procedures and Anticoagulants: What You Need to Know About Bruising and Bleeding Risks
Learn the real risks of cosmetic procedures when you're on blood thinners. Discover which medications are safe to keep, when to pause them, and why stopping them can be more dangerous than continuing.
Asthma in Children: How Spacers, Schools, and Care Plans Work Together
Spacers make asthma inhalers work better for kids, reduce hospital visits, and help them stay in school. Learn how to use them correctly, why schools need them, and how to build a solid asthma care plan.